Camera-based,
non-contact pulse wave
measurement(rPPG)





Measurements are completed within one minute,
significantly reducing testing and waiting time
Fast and convenient registration
and login through non-contact facial recognition
Clearly captures heart rate and HRV signals,
providing a stable foundation for analysis

Expands data coverage by connecting
with external devices such as blood pressure monitors
and body composition analyzers
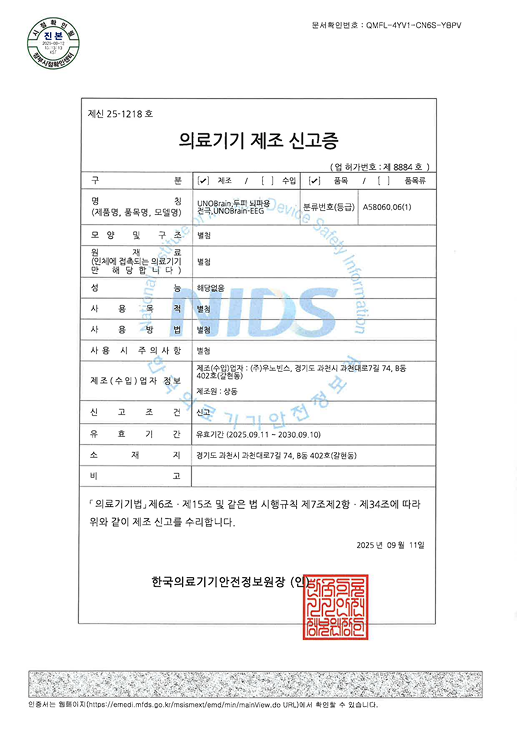
EEG modules can be optionally selected
to expand brainwave-based analysis and reporting
Includes standardized questionnaires
for objective screening and quantitative reporting
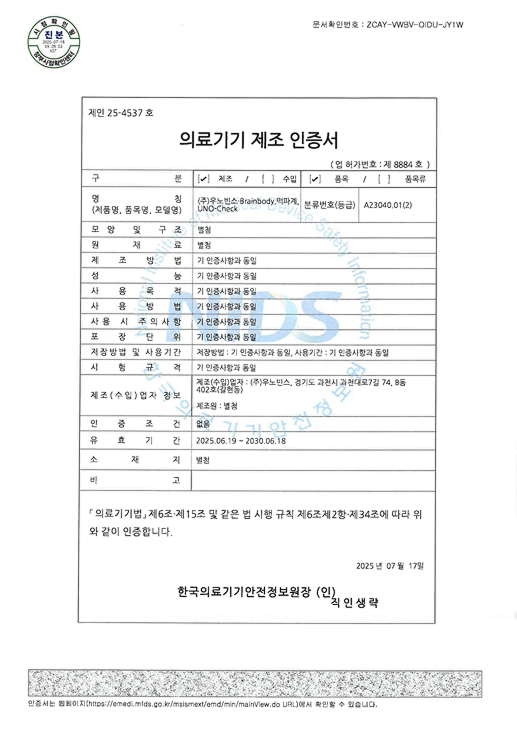
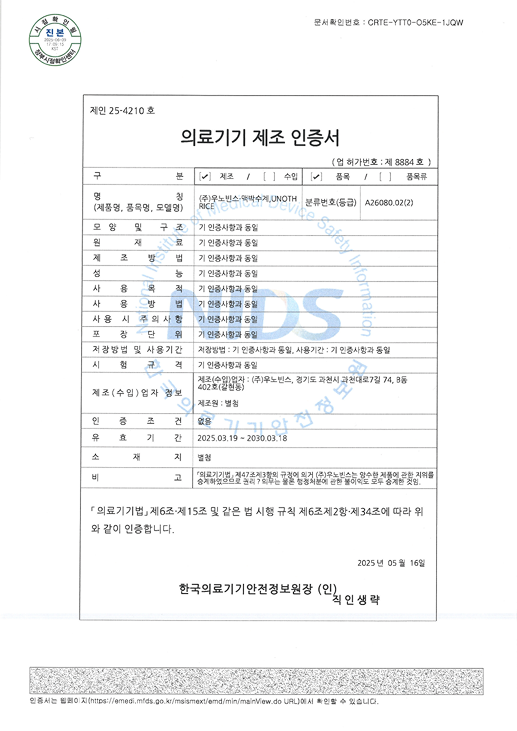
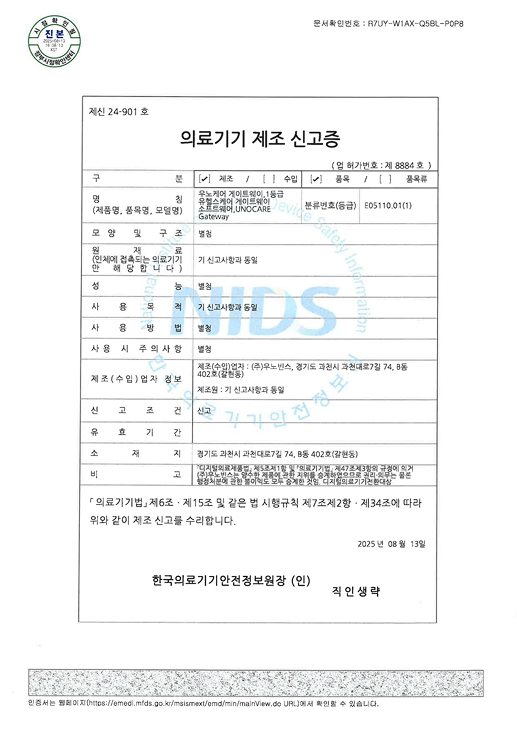
Certified as a Class II medical device by the KFDA,
and designed, manufactured,
and quality-controlled in compliance
with GMP (Good Manufacturing Practice) standards.


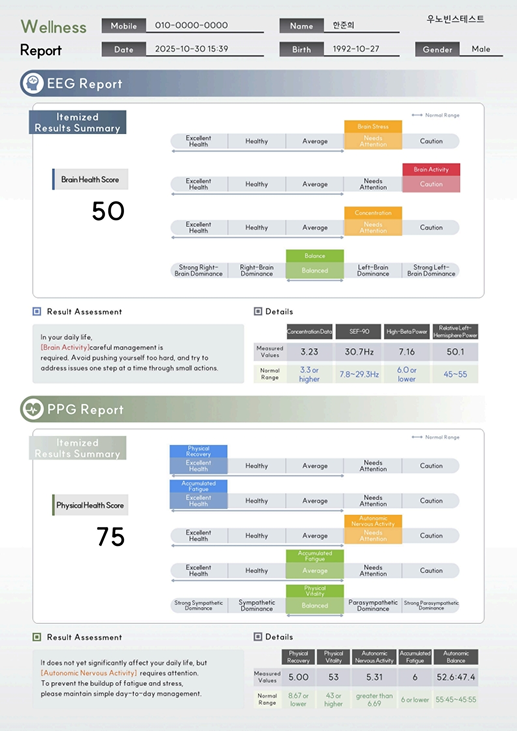
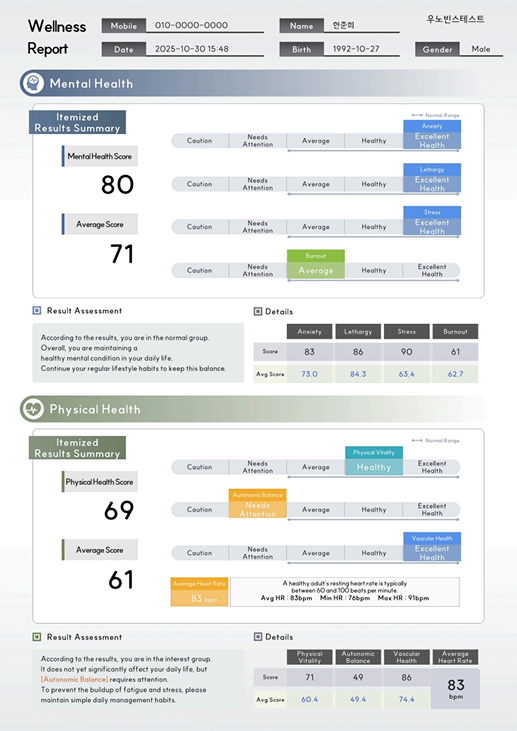
A results report that enables intuitive review
of key indicators such as stress, fatigue,
and vascular health through graphs and scores.


| Item | Specifications |
|---|---|
| Pulse Measurement Device | 1. Model : UNOTHRICE (Certificate No. 25-4210) 2. Channels : 1 Pulse Channel 3. Measurement Method : Optical (Measurement Range : 30–200 BPM) 4. Input Power : 5V, 500mA 5. Interface : USB Type A/C 6. Dimensions : 159 mm (W) × 195 mm (D) × 47 mm (H) 7. Weight : 300 g 8. Protection Type / Degree : External Power Device |
| EEG Measurement Device *Option | 1. Model : MTV-001 (Certificate No. 25-4646) 2. Channels : 2 EEG Channels 3. Measurement Method : Mono-Polar 4. Input Noise Level : ≤ 6 μV 5. Input Power : 3.7 Vdc, 95 mAh 6. Dimensions : 31.5 mm × 27.5 mm × 10.1 mm 7. Weight : Approx. 12 g |
| Measurement Program Display | 1. Device Type : Kiosk-mounted Tablet 2. Model : UNO-ACT (POSBANK) 3. OS : Android 12 4. Display : 15.6inch / IPS 1920*1080, 1080P 5. Communication : WIFI 2.4GHz/5GHz, Bluetooth 4.0, 10/100 Ethernet LAN RJ45 6. CPU : Cortex-A72 / 6core (1.8GHz * 6) 7. Power : DC 12V 5A 8. Dimensions & Weight : 366 mm (W) × 232 mm (D) × 35 mm (H) / 2.95 kg |
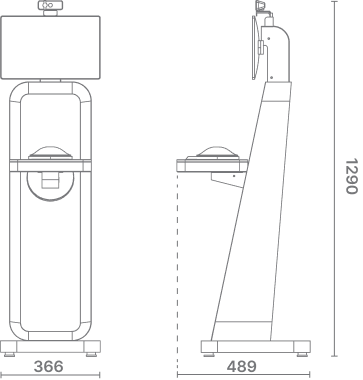
| Main Body | 1. Form Factor : Stand-type Kiosk 2. Material : EGI 1.0t (white painting) 3. Dimensions : 400 mm (W) × 366 mm (D) × 1290 mm (H) |
| Accessories | 1. Power Cable |